How do scientists design anti-cancer drugs that re-activates the immune response ?
The immune system plays a key role in fighting off by tracking down and eliminating cancer cells. Cancer cells, however, also have ways of evading the immune system.Many cancer cells produce in large quantities a proteine known as indoleamine 2,3-dioxygenase 2 (IDO1). A high rate of IDO1 in a tumour is usually linked to poor prognosis. Why? IDO1 degrades tryptophane – an essential amino acid - that has to be in sufficient quantities for cells to divide normally. The high concentration of IDO1in the environment of a cancer cell decreases the amount of tryptophane, which in turn stops immune cells, or T lymphocytes, from proliferating. Furthermore, certain side products which result from tryptophane degradation are toxic for T lymphocytes.
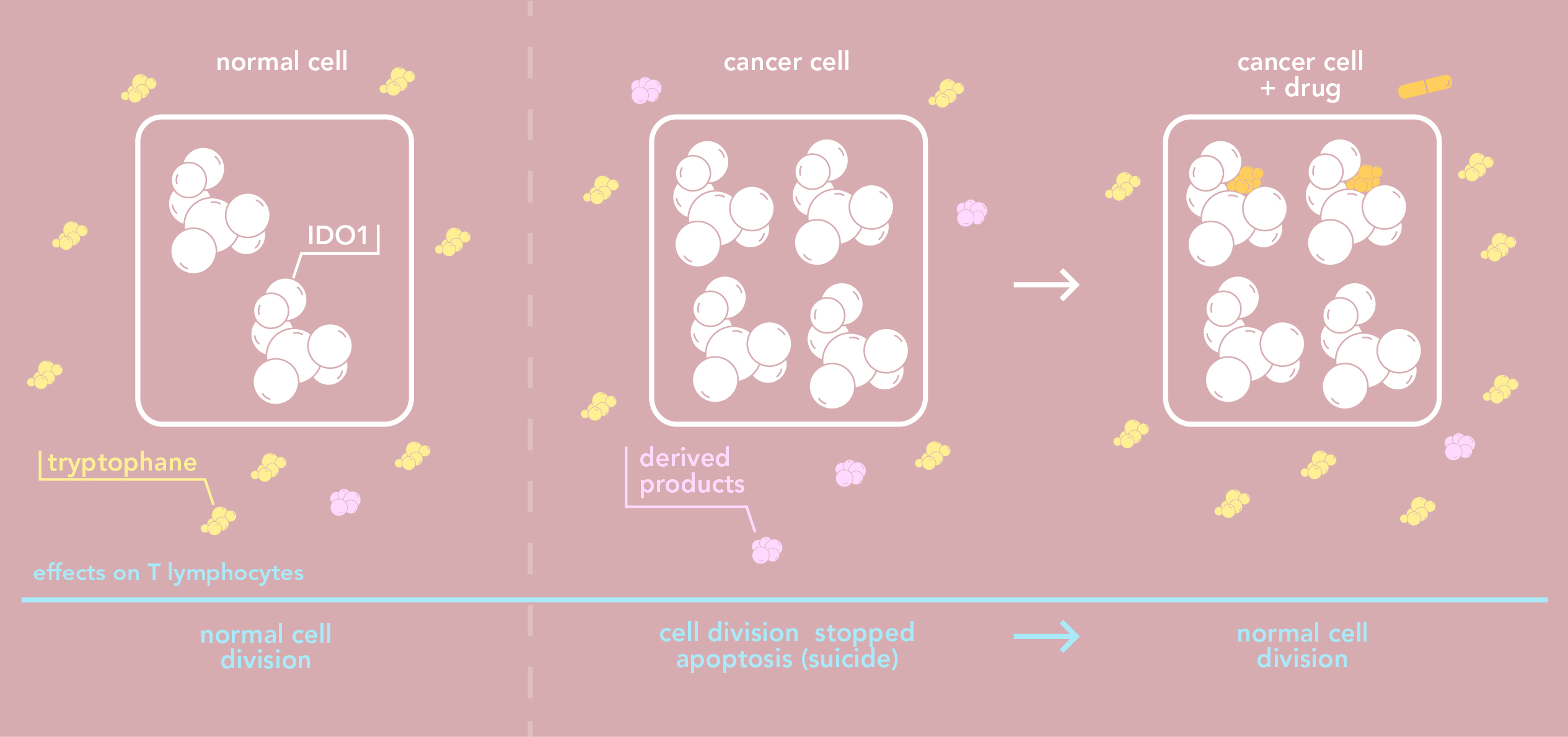
Consequently, when IDO1 is expressed in large quantities, tumours are able to evade the natural immune defense system. IDO1 also helps tumours evade an induced immune defense system as is the case in immunotherapy - a new type of anti-cancer treatment which is given with more traditional treatments such as chemotherapy or radiotherapy. This is why IDO1 is a promising therapeutic target in anti-cancer treatment: inhibiting IDO1 increases the effectiveness of immunotherapies as well as other treatments by restoring the immune response. Since the beginning of the millennium, developing molecules that specifically inhibit IDO1 has now become the focus of intensive research.
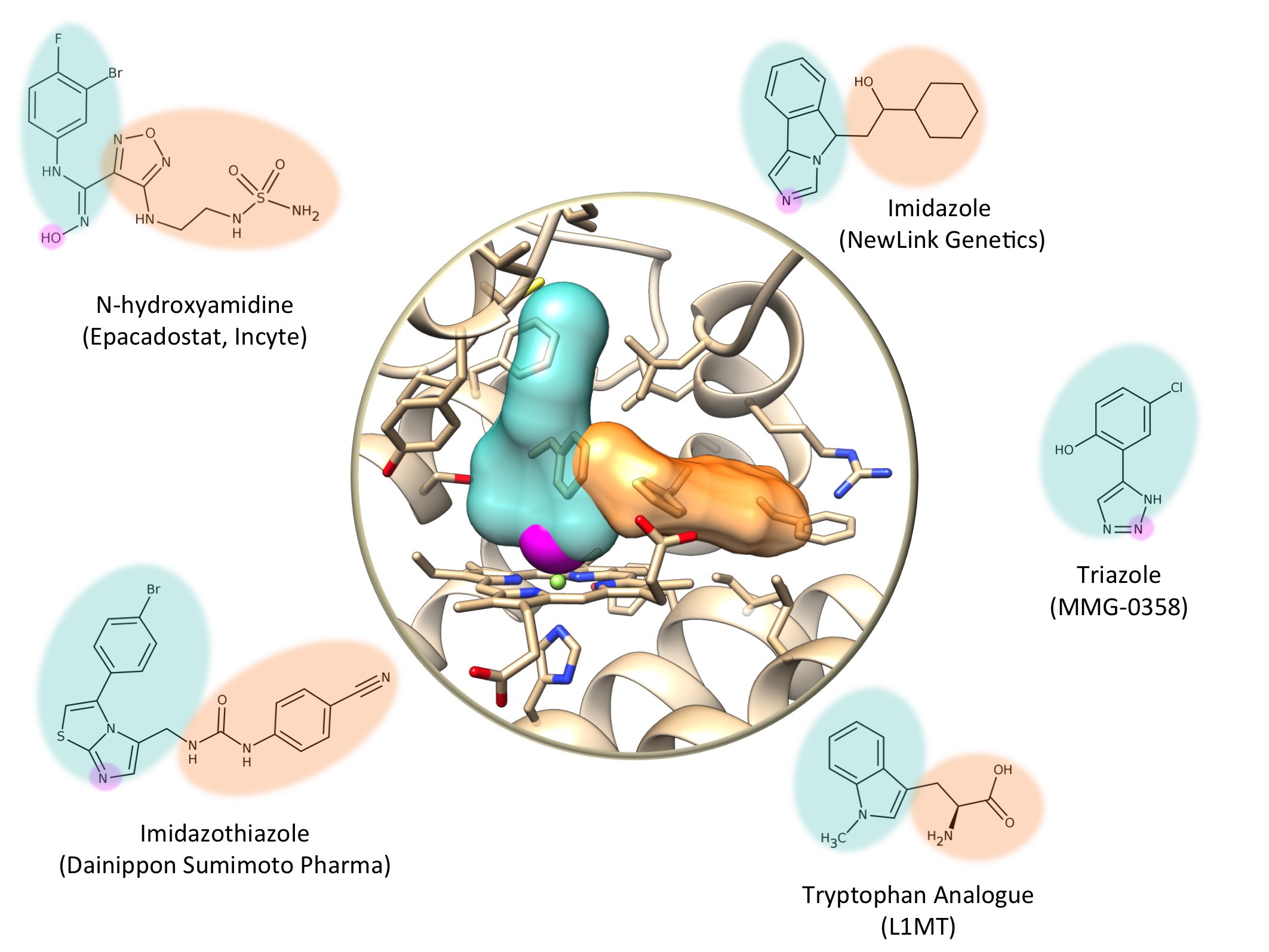
The first IDO1 inhibitors were tryptophane analogs, which are molecules very similar to the natural substrate of the protein. Other scientists discovered inhibitors by screening chemical libraries on cell cultures. The discovery of the 3D structure of IDO1 by crystallography and X-ray diffraction in 2006 paved the way to designing new molecules with the aid of computer models (“computer-aided structure-based drug design). As an example, at the SIB Swiss Institute of Bioinformatics, dozens of molecules derived from triazole – amongst which one known as MMG-0358 – seems very promising.
Here are a few examples of molecules that may well become anti-cancer drugs:
Note : IDO1 is part of many physiological or pathological processes involved in the immune response : chronic infections, inflammation, transplantations, maternal-fœtal tolerance, autoimmune illnesses and allergies. IDO1 inhibitors could also be developed to treat other illnesses besides cancer, such as neurodegenerative illnesses (Alzheimer’s, Parkinson’s, Huntington disease, amyotrophic sclerosis, …) or certain infections (HIV, tuberculosis, …)
This workshop shows how bioinformatics:
- is used to design a molecule that can target IDO1 specifically, by using a technique known as molecular docking. This technique enables to predict the way a molecule binds to a protein (3D structure) as well as its binding force (score calculation),
- can predict one or more of the drug’s target proteins (SwissTargetPrediciton),
- can predict the future of a small molecule in the human body (SwissADME: Absorption, Distribution, Métabolisme et Excretion).
You can compare molecule efficiency, security and specificity of what you have designed with the following molecules:
- AMG-1, an imidazothiazole developed by Dainippon Sunimoto Pharma.
- MMG-0358, a triazole designed by the SIB Swiss Institute of Bioinformatics. MMG-0358 has a strong affinity for IDO1 and is active in vivo.
- PIM, an imidazole. PIM is one of the first inhibitors whose 3D structure complexed with IDO1 has been characterized. PIM has a weak affinity for IDO1 and is not very specific.
- L1MT, or L-1-methyltryptophane, an inert analog of tryptophane that inhibits IDO1. L1MT is undergoing clinical tests for the treatment of breast and prostate cancer.
- NLG-919, an imidazole developed by NewLink Genetics. NLG-919 is under clinical evaluation to treat solid tumors.